ISO/IEC 17025:2017 - A New Accreditation Standard
Contents
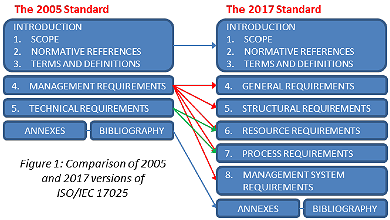
This brief Information Leaflet, prepared by the Eurachem Education and training Working Group, gives a quick overview of the main changes in the 2017 edition of the Standard.
Note: See 'Maintenance' below for further information on continued relevance of this Information Leaflet.
Availability
The leaflet is available in several different languages:
- View/download the leaflet in English (pdf, 399 kB, published 2018-07-16)*
- View/download the leaflet in Bulgarian (pdf, 213 kB, published 2018-07-16)*
- View/download the leaflet in Estonian (pdf, 445 kB, published 2020-02-14)*
- View/download the leaflet in Farsi (pdf, 680 kB, published 2019-07-15)
- View/download the leaflet in Georgian (pdf, 562 kB, published 2018-12-26)*
- View/download the leaflet in Russian (pdf, 530 kB, published 2019-03-18)
- View/download the leaflet in Spanish (pdf, 310 kB, published 2018-07-16)*
- View/download the leaflet in Turkish (pdf, 483 kB, published 2018-07-16)*
* File updated 2024-11-07 to correct link to Eurolab Cookbooks and update references
Translations
Translation into other languages is encouraged for members of Eurachem. Other offers of translation should be directed to the Eurachem Secretariat for permission. The Eurachem policy on maintenance and development of Eurachem guidance, available on the Policies page, gives further information on translation.
Maintenance of this information leaflet
As of 2023, the date of the most recent periodic reviewNote 1 of this Information Leaflet, ISO/IEC 17025:2017 is well established in accredited laboratories. The responsible Eurachem Working Group nonetheless consider that information in the leaflet remains of current interest and have consequently confirmed the leaflet in its original form as current Eurachem guidance.
Note 1. Eurachem policy on maintenance of Eurachem guidance can be found on the "Eurachem Policies and Procedures" page on this website.
- Details
- Last Updated: Friday, 06 February 2026 14:52



