Assessment of performance and uncertainty in qualitative chemical analysis
Introduction
 Many decisions with socio-economic or individual impact depend on qualitative analysis, including decisions related to food safety, clinical diagnosis, and forensic evidence, are based primarily on qualitative, rather than quantitative, chemical analysis. Qualitative analysis is analysis that returns a classification rather than a numerical value, such as the identity of a chemical substance, the type of plastic of a microparticle, the potential source of an oil spill, the presence of a banned sports doping substance, or the presence of accelerant in fire debris. Sometimes, such classifications rely solely on qualitative tests; others may use measurement results (such as line frequencies in a spectrum) to reach a conclusion.
Many decisions with socio-economic or individual impact depend on qualitative analysis, including decisions related to food safety, clinical diagnosis, and forensic evidence, are based primarily on qualitative, rather than quantitative, chemical analysis. Qualitative analysis is analysis that returns a classification rather than a numerical value, such as the identity of a chemical substance, the type of plastic of a microparticle, the potential source of an oil spill, the presence of a banned sports doping substance, or the presence of accelerant in fire debris. Sometimes, such classifications rely solely on qualitative tests; others may use measurement results (such as line frequencies in a spectrum) to reach a conclusion.
Qualitative analysis, like quantitative analysis, needs to be demonstrably reliable. Part of the purpose of the guide is accordingly to show how the performance of a qualitative analysis procedure can be quantified to ensure its fitness for purpose. Practical difficulties and limitations in reliable quantification of low false result rates are discussed, and recommendations are made for checking the validity of these analyses. Brief recommendations are also made for ensuring that any measurements undertaken in the course of a qualitative analysis are reliable.
Although laboratories accredited following the ISO/IEC 17025 standard are not currently required to express qualitative analysis results with uncertainty, the guide provides some metrics that can be used to convey the level of confidence in conclusions from qualitative analysis. In addition, the guide provides suggestions for reporting results with an indication of confidence if desired.
This guide does not make recommendations on whether or not laboratories should routinely report information on the confidence, or uncertainty, associated with qualitative analysis results. Nevertheless, an understanding of performance, or of the uncertainty associated with a qualitative analysis result, can help laboratories to better advise customers; it can also help to identify quality improvements or determine (for example) whether additional confirmatory tests might be needed.
This Eurachem/CITAC guide accordingly aims to provide some tools to help improve the quality of qualitative analysis and of their interpretation, to make sure that interests dependent on these analyses are well protected.
Content of the guide
This guide includes information on
- The nature and relevance of qualitative analysis.
- Different types of qualitative analysis - for example, those that depend on qualitative tests alone, and others that use quantitative information to come to a conclusion.
- Experimental and other strategies for estimating false response rates, which are fundamental to establishing reliability.
- Expressions of confidence in qualitative analysis.
- Reporting qualitative analysis results.
- Examples showing how false response rates and other metrics can be determined.
- The statistical basis for some important measures of confidence in a qualitative result.
Availability
The guide may be downloaded from this website at no cost.
- Download the Guide in Czech (PDF, 1.4 Mb) (Published 2022-12-20)
- Download the Guide in English (PDF, 1.8 Mb) (Published* 2021-11-17)
- Download the Guide in Farsi (PDF, 2.3 Mb) (Published 2024-04-04)
- Download the Guide in German (PDF, 1.4 Mb) (Published 2024-04-04)
- Download the Guide in Russian (PDF, 2.6 Mb) (Published 2024-06-18)
* First published 2021-11-11. Publication dates above are dates of file publication on this website.
Translation
Translation into other languages is permitted for members of Eurachem. Other offers of translation should be directed to the Eurachem Secretariat for permission. The Eurachem policy on maintenance and development of Eurachem guidance, available on the Policies page, gives further information on translation.
Citation
This guidance should be cited* as
"R Bettencourt da Silva and S L R Ellison (eds.) Eurachem/CITAC Guide: Assessment of performance and uncertainty in qualitative chemical analysis. First Edition, Eurachem (2021). ISBN 978-0-948926-39-6.
Available from www.eurachem.org."
*Subject to journal requirements.
Previous editions
There are no earlier editions of this Guide.


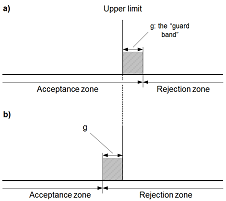 In order to decide whether a result indicates compliance or non-compliance with a specification, it is necessary to take into account the measurement uncertainty associated with the result. This guide provides guidance on how uncertainty may be taken into account in deciding compliance with a limit.
In order to decide whether a result indicates compliance or non-compliance with a specification, it is necessary to take into account the measurement uncertainty associated with the result. This guide provides guidance on how uncertainty may be taken into account in deciding compliance with a limit. Blanks are an important tool and are used in the determination of most performance characteristics during a validation process. They are also often included in each analytical run during routine use of the measurement procedure. There are many different types of blanks and the analyst must consider which blanks to include during preparation of the validation plan.
Blanks are an important tool and are used in the determination of most performance characteristics during a validation process. They are also often included in each analytical run during routine use of the measurement procedure. There are many different types of blanks and the analyst must consider which blanks to include during preparation of the validation plan. Planning is an essential stage in the validation process. Before starting any experimental work, the aim should be to have a clear plan for the entire validation study. This should cover the performance characteristics that will be studied, the target value for each performance characteristic, the materials that will be analysed, the level of replication and order of the experiments, any statistical analysis that will be used, and how the method will be judged as being fit for purpose.
Planning is an essential stage in the validation process. Before starting any experimental work, the aim should be to have a clear plan for the entire validation study. This should cover the performance characteristics that will be studied, the target value for each performance characteristic, the materials that will be analysed, the level of replication and order of the experiments, any statistical analysis that will be used, and how the method will be judged as being fit for purpose.