Guide to Quality in Analytical Chemistry - 4th edition (2026)

Content
 The aim of this guide is to provide laboratories with guidance on best practice for the analytical operations they carry out. The guidance covers both qualitative and quantitative analysis carried out on a routine or non-routine basis.
The aim of this guide is to provide laboratories with guidance on best practice for the analytical operations they carry out. The guidance covers both qualitative and quantitative analysis carried out on a routine or non-routine basis.
This 4th edition is a revision of the CITAC/Eurachem Guide published in 2016. This revision reflects changes that were introduced with the publication of the 2017 version of ISO/IEC 17025.
The Guide focuses on the requirements of ISO/IEC 17025, however the content should also be of use to organisations seeking accreditation or certification against the requirements of standards such as ISO 15189 or ISO 9001, or compliance with the Principles of Good Laboratory Practice. The Guide will also provide useful information both for laboratories that wish to establish a quality management system but are not seeking formal recognition, and for those involved in education and training.
Availability
You may download the guide from this website at no cost.
- Download the guide in English [pdf, 1.6 Mb] (2026-02-06]+
+ Dates of publication on this site
Translations
Translation into other languages is permitted for members of Eurachem. Other offers of translation should be directed to the Eurachem Secretariat for permission. The Eurachem policy on maintenance and development of Eurachem guidance, available on the Policies page, gives further information on translation.
Publication date
This third edition was approved for publication in January 2026 and first published on this website on 6th February 2026.
Citation
This publication should be cited* as: “V. Barwick and K. C. Tsimillis (Eds), Eurachem/CITAC: Guide to Quality in Analytical Chemistry: An Aid to Accreditation (4th ed. 2026). ISBN 978-0-948926-40-2. Available from www.eurachem.org
*Subject to journal requirements
Previous editions
The third edition of this Guide, and earlier editions, can be found in the publication archive on this website.


 Guidance is given on how to validate a measuring procedure that includes the primary sampling (VaMPIS). It is a supplement to existing Eurachem guidance on “The Fitness for Purpose of Analytical Methods” and “Measurement Uncertainty arising from Sampling”. The overall aim is to extend the concept of ‘validation of a measurement procedure’ beyond the validation of the analytical method (or procedure) alone, in order to include the primary sampling (and physical sample preparation) within the validation process.
Guidance is given on how to validate a measuring procedure that includes the primary sampling (VaMPIS). It is a supplement to existing Eurachem guidance on “The Fitness for Purpose of Analytical Methods” and “Measurement Uncertainty arising from Sampling”. The overall aim is to extend the concept of ‘validation of a measurement procedure’ beyond the validation of the analytical method (or procedure) alone, in order to include the primary sampling (and physical sample preparation) within the validation process.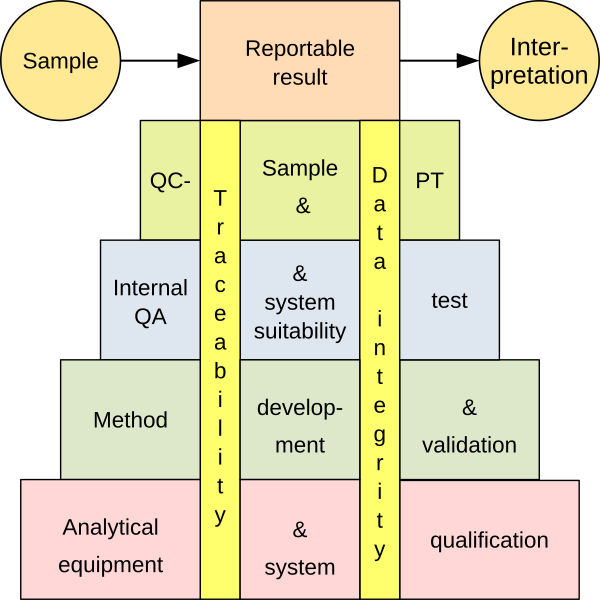 A wide variety of analytical equipment is used in analytical laboratories, ranging from simple apparatus to complex computer-based systems, to collect data that helps to obtain a reportable result. Many of these pieces of equipment combine a measurement function with software control. There are many ways to demonstrate that an equipment is qualified and under control, including qualification, calibration, validation and maintenance. To ensure ‘fitness for purpose’, an integrated approach based on risk assessment is recommended.
A wide variety of analytical equipment is used in analytical laboratories, ranging from simple apparatus to complex computer-based systems, to collect data that helps to obtain a reportable result. Many of these pieces of equipment combine a measurement function with software control. There are many ways to demonstrate that an equipment is qualified and under control, including qualification, calibration, validation and maintenance. To ensure ‘fitness for purpose’, an integrated approach based on risk assessment is recommended.