Recent news
CGPM votes unanimously to change the SI
 At 12:20 CET on November 16, 2018, CGPM, the General Conference on Weights and Measures responsible for decisions on the systeme internationale, voted unanimously to move the foundations of physical and chemical measurement.
At 12:20 CET on November 16, 2018, CGPM, the General Conference on Weights and Measures responsible for decisions on the systeme internationale, voted unanimously to move the foundations of physical and chemical measurement.
The CGPM met, at an open meeting at the Palais des Congrès, Versailles, to discuss and vote on the re-definition of four of the SI’s seven base units: the mole, the ampere, the kelvin, and the kilogram.
This change, which came into effect on World Metrology Day (20th May) 2019, is perhaps the most fundamental change in the SI since its inception. For the first time, the SI will be defined entirely in terms of fundamental physical constants, instead of requiring the maintenance of a physical artefact.
How do the changes remove reliance on physical standards?
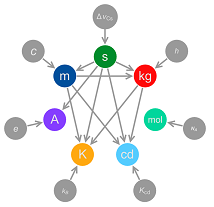
This is achieved by defining the kilogram – currently the last SI unit to be based on a physical artefact – in terms of the Planck constant; the fundamental physical constant that relates frequency to energy. At the same time, the ampere will be defined in terms of the elementary charge e; the unit of temperature, the kelvin, will be defined by reference to the Boltzmann constant and the mole will be defined in terms of the Avogadro constant. In the new SI, all of these constants will take fixed values in SI units, implicitly defining each unit.
Why is this important?
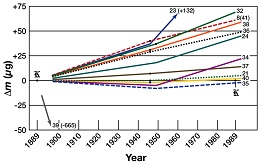
| The most important technical reason for making this momentous change is that reliance on a single physical artefact is simply not sufficiently reliable for the needs of modern and future high-accuracy measurement. The present definition of the kilogram is, essentially, the mass of a particular prototype kilogram held in the vaults of BIPM at Sèvres, near Paris. At the simplest level, if the artefact were ever damaged or lost, the SI would no longer have its anchor for mass. More importantly, measurements over time of the many national kilograms show a degree of drift – due, perhaps, to simple wear – that compromises their long term viability. Redefining the kilogram in terms of a fundamental constant removes the reliance on a single artefact. |
Similarly, defining the ampere in terms of the fundamental charge, via the coulomb, makes it possible to realise the ampere with much improved accuracy. The definition of the kelvin, currently using the triple point of water, will also change fundamentally to include a fixed numerical value of the Boltzmann constant in J K-1 (equivalent to kg m2 s-2 K-1); again, this opens the way for steady improvements in uncertainty for temperature standards.
In wider terms, it becomes possible – at least in principle – for every measurement institute to realise their own units of mass, and all the other SI units, using fundamental physics instead of relying on comparison with a single artefact. The foundations of measurement are now truly available to all.
What is the scientific basis of the redefinition?
 These changes are the culmination of decades of development work, most recently related to the development of the ‘Kibble balance’, and the detailed work in the Avogadro project to determine the Avogadro constant to unprecedented accuracy.
These changes are the culmination of decades of development work, most recently related to the development of the ‘Kibble balance’, and the detailed work in the Avogadro project to determine the Avogadro constant to unprecedented accuracy.
The Kibble balance is a device that allows a direct comparison of force generated by an electric field against the gravitational force on a standard mass. Previously, this phenomenon was used to realise the ampere; but with steady improvements in the measurement uncertainty, it is now possible to relate the force generated to fundamental constants including the Planck constant and the charge on the electron. Defining these constants as fixed values in SI units is equivalent to defining the units themselves.
The Avogadro constant – a fundamental feature of any chemistry course – has been measured to extraordinary accuracy as part of the Avogadro project. The scientific principle is relatively simple; by measuring the size of a unit cube in pure silicon using crystallography, and combining this with accurate density measurement and the known atomic mass of silicon, the number of atoms in a mole of silicon can be determined. Silicon was chosen because it is available in extremely high purity; even so, it was essential to use very highly enriched silicon-28 to ensure that uncertainties associated with isotopic impurities – as well as surface oxide and other impurities – were sufficiently small to determine the constant to nine significant digits.
Further, since the measurement of the Avogadro constant also relied on mass, it was possible to cross-check the Avogadro project results against those from work on the Kibble balance; the redefinition was not enacted until agreement was so good that no practical measurement would suffer any adverse impact from the change.
What difference will it make to practical chemical and biological measurements?
In practice, the changes will make no difference at all to any practical measurement. The fixed values chosen for the fundamental constants are carefully aligned with the best available values, so that there is no visible change. The uncertainties are far, far smaller than any realistic chemical measurement uncertainty currently available. Chemical and biological measurement scientists will see “business as usual” for the mole. Calibration and accreditation will carry on as usual; we will not need to recalibrate any instruments or review accreditation as a consequence of the change.
In the longer term, there could be small changes in high-accuracy measurements of atomic mass. Since the mole will no longer be reliant on the kilogram and the atomic mass of carbon 12, the dalton – a convenience unit currently defined as 1/12 times the mass of an atom of carbon-12 – will no longer have a fixed relationship to all three of the kilogram, the Avogadro constant NA, and the mass of 12C. Instead, the dalton is likely to be defined to retain its relationship to the mass of 12C, with the consequence that exactly 1 g of 12C will no longer contain identically NA atoms by definition. It currently seems unlikely that this will have important ramifications in practice, as the relationship will hold within the very small uncertainties involved.
Perhaps a more exciting possibility is that the changes may usher in a new generation of high accuracy measurement instruments for other units on which chemists rely. For example, once the kelvin is decoupled from the traditional water triple point cell, new instruments for very high accuracy measurement of temperature, based on different physical principles, become conceivable. Such developments could open a route to greater accuracy, or greater simplicity, for a wide range of measurements that are routinely undertaken in the course of chemical and biological measurement.
How will CIPM and the national measurements guarantee comparability of their standards if they are no longer traceable to the same physical artefacts?
Calibrations for most measurement standards will not change in the short term, and the general principles will still apply within each country. To make sure measurements are consistent across borders, there is already a well-established system of international comparisons; the CIPM Mutual Recognition Arrangement (see https://www.bipm.org/en/cipm-mra/). Although some national laboratories may transfer their primary mass standard calibrations to instruments based on the Kibble balance, they will still be able to weigh and compare standards across borders to make sure that their measurements remain compatible.
Summary
The CGPM, with the support of decades of work by measurement scientists in national laboratories world-wide, have taken a historic decision that will improve the stability of measurement world-wide and in decades to come.
The change will, for the first time, made the whole of the SI universally available. The new definitions, no longer dependent on any particular artefact, will make the fundamental units of physical and chemical measurement more stable in the very long term, setting a firm foundation for future decades of measurement. Comparability across national borders will continue to be maintained by laboratories participating in the CIPM MRA. Although fundamental, the changes will have no adverse effects on day-to-day analytical measurement, and calibration and accreditation activities will continue unperturbed; no calibration certificates will need to be renewed earlier than usual. This is a great achievement for metrology and for all of science.
More information
BIPM videos related to the new SI: https://www.youtube.com/thebipm
NIST article: https://www.nist.gov/si-redefinition/turning-point-humanity-redefining-worlds-measurement-system
NPL, UK article: http://www.npl.co.uk/si-units/redefining-the-si-units/
Author: S Ellison
Chair, Eurachem MU and Traceability WG
2018-11-16
Note: This article has been updated slightly from the original News article, to include the date of the CGPM decision
- Details
- Published: Friday, 16 November 2018 14:52