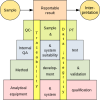
Image courtesy of SP, Sweden
Welcome to Eurachem, a network of organisations in Europe having the objective of establishing a system for the international traceability of chemical measurements and the promotion of good quality practices. It provides a forum for the discussion of common problems and for developing an informed and considered approach to both technical and policy issues. It provides a focus for analytical chemistry and quality related issues in Europe.
Eurachem e-News
For email updates about Eurachem, you can subscribe to our mailing list.